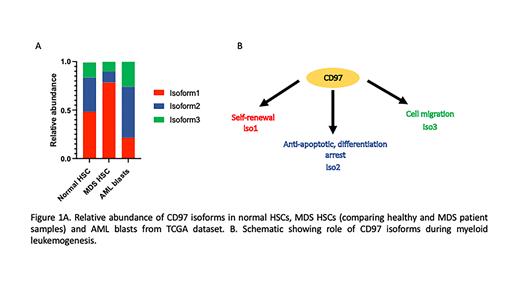
CD97 is an adhesion GPCR protein frequently upregulated in AML and required for LSC self-renewal that is alternatively spliced to form three isoforms that are distinct with respect to their extracellular domain. Our analysis of HSCs from MDS patients with low-risk disease revealed overexpression of CD97 isoform 1 (CD97iso1), while analysis of AML samples from the TCGA dataset revealed preferential expression of CD97iso2 (p<0.0001) (Figure1A). As these data suggested potential isoform specific roles of CD97 in myeloid leukemogenesis, we investigated if the different CD97 isoforms support different functional roles by testing the effect of each isoform on growth, differentiation, and clonogenicity of AML blasts. Consistent with our prior studies demonstrating CD97iso1 promotion of self-renewal in normal CD34+ hematopoietic stem/progenitor cells, over expression of CD97iso1 in AML cell lines increased their serial replating ability, while knockout abrogated their clonogenicity. In stark contrast, overexpression of CD97iso2 or CD97iso3 resulted in decreased clonogenicity upon replating. Overexpression of CD97iso2 conferred the greatest growth advantage to AML cells among the CD97 isoforms. Importantly, knockout of CD97iso2 increased cell differentiation and primed the cells towards apoptosis. Supporting CD97iso2's role in cell growth/survival, daunorubicin treatment increased the expression of CD97iso2. Consistent with CD97's role in increasing migration and invasiveness in solid tumors, we performed trans well migration assays using AML cell lines and observed the highest increase in migration in blasts over expressing CD97iso3 (p<0.01).
While CD97 is thought to activate several downstream signaling pathways including the MEK-ERK pathway and PI3K/AKT pathway, it is unknown whether the different CD97 isoforms activate unique downstream signaling pathways, and thus we used an unbiased Bio-ID screen to identify the C-terminal interacting partners of the different CD97 isoforms. We observed preferential enrichment of NLRX1 interactions in CD97iso1 expressing cells, while PI4KA was enriched in CD97iso2 expressing cells. Consistent with NLRX1's role in increasing autophagy, we observed an increase in autophagy upon overexpressing CD97iso1, and consistent with CD97iso2's association with PI4KA, CD97iso2 overexpressing cells showed increased PI4KA and phosphoERK1/2. Finally, confirming non-redundant functional roles of CD97 isoforms in vivo, xenograft studies in NSG mice using AML cell lines revealed that CD97iso2 promoted the most rapid engraftment and shortest survival in recipients (p=0.0001). Given the importance of all the CD97 isoforms in regulating different blast function, we developed antibody-drug conjugates (ADCs) based on human synthetic antibodies that target all isoforms of CD97. ADC against CD97 specifically kills AML cells overexpressing CD97, suggesting the potential use of anti-CD97 ADC for AML therapy.
Collectively, these studies demonstrate that CD97 isoforms mediate unique biological roles in vitro and in vivo, and that they likely mediate these functions through differential activation of downstream signaling pathways. We speculate that differential expression of the CD97 isoforms in MDS and AML likely reflects the unique features of MDS HSCs (high levels of CD97iso1 enhancing self-renewal in MDS) versus AML LSCs (CD97iso2 promoting proliferation in AML) (Figure 1B). These studies also highlight the importance of isoform-specific gene expression during myeloid leukemogenesis and suggest that explorations of altered isoform expression in MDS/AML will yield novel insights into the molecular mechanisms that promote disease development and progression.
Disclosures
Koide:Argenx: Patents & Royalties: Intellectual Property Licensing, Research Funding; Black Diamond Therapeutics: Research Funding; Puretech Health: Patents & Royalties: Intellectual Property Licensing, Research Funding; Absolute Antibody: Patents & Royalties: Intellectual Property Licensing; Diagenode: Patents & Royalties: Intellectual Property Licensing; Millipore: Patents & Royalties: Intellectual Property Licensing.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal